Biology 403, Thirteenth Lecture
Tuesday 9 March 2004
Carbohydrate Metabolism
Outline
- What happens in glycolysis
- Why it's important
- The ten enzymatic steps
- The fate of pyruvate
- Free energy in glycolysis
- Regulation of glycolysis
- Other sugars in glycolysis
- Bisphosphoglyerate in erythrocytes
- Gluconeogenesis
- Pentose phosphate shunt
What happens in glycolysis
Glycolysis is the process whereby glucose is converted to pyruvate
in ten enzymatic steps. This process is catabolic; i.e., it involves
breakdown of a molecule into smaller pieces, and as is typical of catabolic
processes, it results in the net production of ATP. There isn't a lot
of ATP produced in glycolysis: just two molecules of ATP are produced
per molecule of glucose input. Much more ATP is produced in the Krebs
cycle steps that we will study in a couple of days. But since pyruvate
is an essential starting point in that cycle, the process we're describing
here leads the way to that energy-rich process.
Pyruvate is also a precursor to fatty acids and other metabolites,
so the conversion of glucose to pyruvate has significance in that regard
as well as its role in energy generation. Furthermore, the process produces
two molecules of reduced NAD per input glucose molecule, so there is
reducing power as well as energy generated in these steps.
Glycolysis includes some phosphorylation steps, which require
energy. Thus the path from glucose to pyruvate is not all downhill;
some steps require ATP, whereas others liberate ATP. The net result,
though, is release of two molecules of ATP per glucose:
Glucose + 2 ADP + 2 NAD+ + 2P
i -> 2 Pyruvate + 2 ATP + 2 NADH + 2H+ + 2H
2 O
The table below is a summary of the reactions involved. Note
that a central step in the process, the one catalyzed by aldolase, involves
converting a 6-carbon bisphosphorylated sugar into two 3-carbon phosphorylated
sugars. This is a typical catabolic reaction for saccharides. In the
table, "E.C. number" refers to the enzyme commission code for the enzyme;
"Resolution" refers to the highest (or nearly the highest) resolution structure
available for the protein in question; "PDB code, yr" refers to the Protein
Data Bank accession code for that highest-resolution structure, and the
year in which that structure was submitted.
Enzymes in the Glycolytic Pathway
Enzyme
|
Reactants
|
Products |
E.C.
number |
Reso-
lution
|
PDB code,
yr |
Cofactors,
cosubstrates |
#aa/
su |
# su |
Hexokinase
|
gluc
|
gluc-6-P
|
2.7.1.1
|
1.9Å
|
1CZA 1999
|
ATP, Mg2+
|
917
|
1,2
|
Phosphoglucomutase
|
gluc-1-P
|
gluc-6-P
|
5.4.2.8
|
1.75Å
|
1K2Y 2001
|
Zn2+
|
463
|
1
|
Phosphoglucose
isomerase
|
gluc-6-P
|
fruc-6-P
|
5.3.1.9
|
1.62
|
1IAT 2001
|
|
557 |
2
|
Phosphofructokinase
|
fruc-6-P
|
fruc 1,6-bisP
|
2.7.1.11
|
2.4Å
|
1PFK 1988
|
ATP, Mg2+
|
320
|
4
|
Aldolase
|
fruc-1,6-bisP
|
glyc3-P, DHA-P
|
4.1.2.13
|
1.67Å
|
1ADO 1996
|
|
363
|
4
|
Triosephosphate
isomerase
|
DHA-P
|
glyc3-P
|
5.3.1.1
|
1.9Å
|
1YPI 1991
|
|
247
|
4
|
Glyceraldehyde-3-P dehydrogenase
|
glyc3-P
|
1,3-bisP glya
|
1.2.1.12
|
1.8Å
|
1GD1 1987
|
NAD, P
|
344
|
4
|
Phosphoglycerate
kinase
|
1,3-bisP glya
|
3-P-glya
|
2.7.2.3
|
1.6Å
|
16PK 1998
|
ATP, Mg2+
|
415
|
1
|
Phosphoglycerate mutase
|
3-P-glya
|
2-P-glya
|
5.4.2.1
|
1.25Å
|
1E58 2000 |
|
249 |
1-4
|
Enolase
|
2-P-glya
|
P-enolpyr
|
4.2.1.11
|
1.8Å
|
1ONE 1995
|
Mg2+
|
436
|
2
|
Pyruvate kinase
|
P-enolpyr
|
pyr
|
2.7.1.40
|
1.8Å
|
1E0T 2000
|
ATP, Mg2+
|
470
|
4
|
Abbreviation
|
Meaning
|
su
|
subunit (monomer)
|
gluc
|
glucose
|
fruc
|
fructose
|
P
|
phosphate, phospho-
|
glyc
|
glyceraldehyde
|
DHA
|
dihydroxyacetone
|
glya
|
glycerate
|
pyr
|
pyruvate |
ATP
|
adenosine triphosphate
|
NAD
|
nicotinamide adenine dinucleotide
|
Some of the enzymes have names that are emblematic of the reverse
reactions, not the reactions as written here, namely, phosphoglycerate
kinase and pyruvate kinase.
To really get a sense of what is happening in these reactions,
you should look at the structures of the small molecules involved in
each of these steps. This graphic is taken from a website at the University
of Texas:

Glycolysis is characteristic of catabolic pathways for sugars in
that it breaks a 6- (or, in other instances, 5-) carbon sugar down into
two approximately equal-sized parts. The actual carbon-carbon bond breakage
occurs at the aldolase step; the other steps involve phosphorylations, dephosphorylations,
and redox reactions. The enzyme ribulose bisphosphate carboxylase / oxygenase
(RuBisCO) is part of an analogous pathway. It disrupts a carbon-carbon
bond in a doubly phosphorylated sugar (similar to fructose 1,6-bisphosphate
in glycolysis) to produce either a three-carbon sugar and a two-carbon
compound or two three-carbon sugars:
ribulose 1,5-bisphosphate + O2 -> 2-phosphoglycolate
+ 3-phosphoglycerate + 2 H+
ribulose 1,5-bisphosphate + CO2 + H2
O -> 2 3-phosphoglycerate + 2H+
The first of these reactions is part of photorespiration, i.e.
the consumption of oxygen in photosynthetic leaves. The second actually
fixes--that is, pulls from the air or water--inorganic carbon in the
form of carbon dioxide or bicarbonate. It is the principal source by which
carbon is incorporated into molecular skeletons. We'll study these reactions
in greater detail in chapter 15, but we note now the similarity in terms
of the sugar bisphosphate's fate to that found in the aldolase reaction.
Why it's important
As we said, these steps produce:
- Energy in the form of ATP; this is used as fuel for many other
reactions.
- Reducing power in the form of NADH; this is required for oxidation-reduction
reactions.
- Pyruvate, which is a significant starting point both for the
Krebs cycle and for lipid biosynthesis.
The ten enzymatic steps
Let's look at the reactions in a bit more detail.
- Hexokinase transfers the γ-phosphoryl group of ATP to
the oxygen atom at C-6 of glucose, producing glucose 6-phosphate and ADP.
This is an instance where the coupling between ATP hydrolysis and an
energy-requiring reaction is very close, because the phosphate is transferred
directly from ATP to the recipient molecule, in this case glucose. Most
enzymes that carry out a reaction of this kind have the word "kinase" at
the end of their name.
The reaction catalyzed by hexokinase is energetically favored:
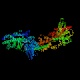 ΔG0 ~ -5.33 kcal/mol, so at 310K (human body temperature)
ΔG0 ~ -5.33 kcal/mol, so at 310K (human body temperature)
Keq = exp(-ΔG0/RT)
= exp(5.33 kcal/;mol / [(1.987 * 10-3 kcal./mol-deg) * 310deg)]
= exp(5.33/(1.987*0.31)) = 5700, so under conditions of adequate
ATP, the equilibrium will definitely favor the product (glucose 6-phosphate)
over glucose.
There are various isozymes (functionally related but structurally slightly
distinct) forms of hexokinase in humans; the liver
form has a Km in the millimolar range, perhaps a factor of 1000
higher than the Km of the hexokinase found in other tissues.
The liver form is therefore much less active than the other forms unless
the liver glucose concentration is high. Hexokinase is active on sugars besides
glucose; the activity against maltose is comparable to the activity on glucose.
Hexokinase has the highest molecular mass per monomer of any of the glycolytic
enzymes; given that it is the first enzyme in an important pathway, it makes
sense that it is large and complex.
- Phosphoglucomutase or phosphoglucose isomerase
interconverts two phosphorylated forms of glucose--glucose 1-phosphate
and glucose 6-phosphate.
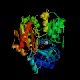
The intermediate is bisphosphorylated, and the equilibrium between the
1-phosphate and 6-phosphate forms is determined by relative concentrations.
This enzyme is active on other phosphorylated aldoses in addition to glucose.
Note that this enzyme does not appear on the chart above, because it is not
part of the linear pathway from glucose to pyruvate.
- Phosphohexoseisomerase or glucose 6-phosphate isomerase
interconverts two monophosphorylated sugars--glucose 6-phosphate and
fructose 6-phosphate.
 As discussed earlier, this interconversion proceeds through a (1,2) ene-diol
intermediate; with the enzyme present the energy barriers around this ene-diol
are lowered enough to speed the interconversion. This dimeric enzyme plays
roles extracellularly as well as intracellularly: it can function as a
nerve growth factor. Each monomer contains two unequal-sized domains, and
the active site is formed by the association of the two subunits.
As discussed earlier, this interconversion proceeds through a (1,2) ene-diol
intermediate; with the enzyme present the energy barriers around this ene-diol
are lowered enough to speed the interconversion. This dimeric enzyme plays
roles extracellularly as well as intracellularly: it can function as a
nerve growth factor. Each monomer contains two unequal-sized domains, and
the active site is formed by the association of the two subunits.
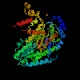
- Phosphofructokinase catalyzes phosphorylation at the 1 position
of fructose 6-phosphate.
 It is an example of a kinase that acts on an already-phosphorylated form,
creating a bisphosphorylated compound. Of all the enzymes in this
pathway it appears to be the one for which the least structural information
is available; note that the best structure determined to date was Phil
Evans's 2.4 Å structure from 1988, and there have not been many other
structures done. ADP acts as an allosteric activator on this enzyme as
well as being a product of the reaction.
It is an example of a kinase that acts on an already-phosphorylated form,
creating a bisphosphorylated compound. Of all the enzymes in this
pathway it appears to be the one for which the least structural information
is available; note that the best structure determined to date was Phil
Evans's 2.4 Å structure from 1988, and there have not been many other
structures done. ADP acts as an allosteric activator on this enzyme as
well as being a product of the reaction.
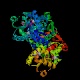
- Aldolase catalyzes the actual carbon-carbon bond cleavage step.
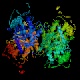 This is a large and important enzyme, and structure determinations began
(unsuccessfully) more than 25 years ago. Some bacterial and yeast forms require
a divalent cation as a cofactor, but the eukaryotic aldolases do not. The
non-cationic forms proceed through an imine (Schiff-base) intermediate. The
enzyme is active on fructose 1-phosphate as well as its "standard" substrate,
fructose 1,6-bisphosphate; in this context it forms part of the catabolic
pathway by which fructose itself can be used as an energy and carbon source.
This is a large and important enzyme, and structure determinations began
(unsuccessfully) more than 25 years ago. Some bacterial and yeast forms require
a divalent cation as a cofactor, but the eukaryotic aldolases do not. The
non-cationic forms proceed through an imine (Schiff-base) intermediate. The
enzyme is active on fructose 1-phosphate as well as its "standard" substrate,
fructose 1,6-bisphosphate; in this context it forms part of the catabolic
pathway by which fructose itself can be used as an energy and carbon source.
- Triosephosphate isomerase (sometimes written with a space after
"triose",
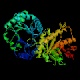 and for some reason abbreviated "TIM") is possibly the most efficient
enzyme known,
in terms of the rate acceleration afforded by the enzyme relative
to the uncatalyzed reaction. It is a tetrameric enzyme with a characteristic
structure in which alpha helical stretches alternate with beta strands such
that the beta strands curve around to form a barrel-like structure with
the helices outside. This structural motif appears in many other enzymes,
and has become known as a "TIM barrel."
and for some reason abbreviated "TIM") is possibly the most efficient
enzyme known,
in terms of the rate acceleration afforded by the enzyme relative
to the uncatalyzed reaction. It is a tetrameric enzyme with a characteristic
structure in which alpha helical stretches alternate with beta strands such
that the beta strands curve around to form a barrel-like structure with
the helices outside. This structural motif appears in many other enzymes,
and has become known as a "TIM barrel."
- Glyceraldehyde 3-phosphate dehydrogenase is a
medium-sized tetrameric enzyme,
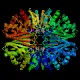 responsible for the conversion of its substrate to
1,3-bisphosphoglycerate.
It resembles several other tetrameric NAD-dependent oxidoreductases, like
lactate dehydrogenase, alcohol dehydrogenase and malate dehydrogenase;
all have characteristic structures in the NAD-binding region known as
"Rossmann folds", after Michael Rossmann,
who first characterized this class of enzymes structurally.
The enzyme is somewhat allosteric.
responsible for the conversion of its substrate to
1,3-bisphosphoglycerate.
It resembles several other tetrameric NAD-dependent oxidoreductases, like
lactate dehydrogenase, alcohol dehydrogenase and malate dehydrogenase;
all have characteristic structures in the NAD-binding region known as
"Rossmann folds", after Michael Rossmann,
who first characterized this class of enzymes structurally.
The enzyme is somewhat allosteric.
- Phosphoglycerate kinase catalyzes the dephosphorylation of 1,3-bisphosphoglycerate.
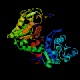 It is named for the reaction running in the opposite direction relative
the one shown in the chart and table above. In the direction shown in the
table it produces ATP rather than consuming it. This enzyme has been shown
to have a hinge motion about a point near the center of the molecule; the
open and closed forms of the enzyme involve movements as large as 17Å
in the residues farthest from the hinge point. This enzyme is primarily alpha-helical
in conformation.
It is named for the reaction running in the opposite direction relative
the one shown in the chart and table above. In the direction shown in the
table it produces ATP rather than consuming it. This enzyme has been shown
to have a hinge motion about a point near the center of the molecule; the
open and closed forms of the enzyme involve movements as large as 17Å
in the residues farthest from the hinge point. This enzyme is primarily alpha-helical
in conformation.
- Phosphoglycerate mutase interconverts 3-phosphoglycerate and 2-phosphoglycerate.
 According to Mathews's
textbook,
According to Mathews's
textbook,
The mechanism of the reaction catalyzed by
phosphoglycerate mutase involves formation of 2,3-bisphosphoglycerate
via transient phosphorylation of a histidine residue of the enzyme.
2,3BPG can diffuse from phosphoglycerate mutase, however,
leaving the enzyme trapped in an unusable state.
Cells make excess 2,3BPG (using the enzyme bisphosphoglycerate mutase)
in order to drive 2,3BPG back to phosphoglycerate mutase,
so the reaction can go to completion.
- Enolase converts 2-phosphoglycerate to phosphoenolpyruvate (PEP).
 This reaction plays a role in gluconeogenesis as well as glycolysis.
Mg2+ ions are required for activity, at least in some forms of
the enzyme. Vertebrate genes code for two slightly different forms of the
monomer of enolase, alpha and beta. Most of the enolase in fetal tissue is
alpha-alpha; mature skeletal muscle contains beta-beta; some alpha-alpha remains
in smooth muscle tissue.
This reaction plays a role in gluconeogenesis as well as glycolysis.
Mg2+ ions are required for activity, at least in some forms of
the enzyme. Vertebrate genes code for two slightly different forms of the
monomer of enolase, alpha and beta. Most of the enolase in fetal tissue is
alpha-alpha; mature skeletal muscle contains beta-beta; some alpha-alpha remains
in smooth muscle tissue.
- Pyruvate kinase is the last enzyme in the pathway.
 It transfers a phosphate from phosphoenolpyruvate to ADP, producing pyruvate
and ATP. The reaction is essentially irreversible. Fructose 1,6-bisphosphate,
the substrate for the aldolase reaction, is an activator of this enzyme,
affording a level of control, known as "feed-forward activation,"
over glycolysis.
Four isozymes of pyruvate kinase are found in humans, derived from two genes.
The fetal (M1) isozyme is nonregulated;
the allosterically regulated forms predominant in adults.
It transfers a phosphate from phosphoenolpyruvate to ADP, producing pyruvate
and ATP. The reaction is essentially irreversible. Fructose 1,6-bisphosphate,
the substrate for the aldolase reaction, is an activator of this enzyme,
affording a level of control, known as "feed-forward activation,"
over glycolysis.
Four isozymes of pyruvate kinase are found in humans, derived from two genes.
The fetal (M1) isozyme is nonregulated;
the allosterically regulated forms predominant in adults.
The fate of pyruvate
If oxygen is abundant, pyruvate is ordinarily converted to acetyl coenzyme
A, and that serves as an entry point into the tricarboxylic acid (citric acid,
or Krebs) cycle. With oxygen available, the NADH that has been produced in
the glyceraldehyde 3-phosphate dehydrogenase step becomes reoxidized
to NAD with concomitant release of energy. We'll discuss that in detail next
week. But if oxygen is scarce, a different pathway known as fermentation,
in which pyruvate is converted to lactate, predominates.
 The enzyme that catalyzes this conversion, lactate dehydrogenase,
is a tetrameric, NAD-dependent enzyme with a molecular mass around
35kDA per subunit--that is,
it is distinctly similar to glyceraldehyde 3-phosphate dehydrogenase.
It catalyzes the reaction
The enzyme that catalyzes this conversion, lactate dehydrogenase,
is a tetrameric, NAD-dependent enzyme with a molecular mass around
35kDA per subunit--that is,
it is distinctly similar to glyceraldehyde 3-phosphate dehydrogenase.
It catalyzes the reaction
pyruvate + NADH + H+ <-> lactate + NAD
so the name is derived from the reverse reaction. An alternative name for
this enzyme would be "pyruvate reductase". This is a zinc-dependent enzyme
, and several structures have been determined for it.
In the absence of oxygen in yeast, a different pathway is followed.
Free energy in glycolysis
Examine carefully fig. 11.12 in Horton. The point it makes is that,
although the standard free energies associated with the various reactions
in glycolysis vary widely, the true free energy changes are monotonically
negative and rather small as we go from glucose to pyruvate.In particular,
there are really only three steps in the process that are effectively
irreversible: the first, third, and last steps, i.e. the hexokinase,
phosphofructokinase, and pyruvate kinase steps. All the others have
ΔG values close to zero. So the only steps
that are irreversible are the ones that involve formation or breakage
of high-energy phosphate bonds. The difference between free energy and
standard free energy is one we emphasized in the previous chapter.
In this instance, the relative abundances of the various metabolites
involved in glycolysis drives the reactions whose ΔGo'
values are positive toward the right.
Regulation of glycolysis
This brings up a related point: irreversible reactions tend to be
the reactions for which control mechanisms come into play.
Horton offers a description of hexose transporters, which are
proteins involved in moving hexoses around from one cell to another.
There are also control mechanisms that operate by inhibition of
specific enzymes in the pathway. In glycolysis, the enzymes on which
inhibitory controls are exerted are the three kinase steps discussed above.
Other sugars in glycolysis
Bisphosphoglyerate in erythrocytes
Gluconeogenesis
The pentose phosphate shunt
 ΔG0 ~ -5.33 kcal/mol, so at 310K (human body temperature)
ΔG0 ~ -5.33 kcal/mol, so at 310K (human body temperature)
 As discussed earlier, this interconversion proceeds through a (1,2) ene-diol
intermediate; with the enzyme present the energy barriers around this ene-diol
are lowered enough to speed the interconversion. This dimeric enzyme plays
roles extracellularly as well as intracellularly: it can function as a
nerve growth factor. Each monomer contains two unequal-sized domains, and
the active site is formed by the association of the two subunits.
As discussed earlier, this interconversion proceeds through a (1,2) ene-diol
intermediate; with the enzyme present the energy barriers around this ene-diol
are lowered enough to speed the interconversion. This dimeric enzyme plays
roles extracellularly as well as intracellularly: it can function as a
nerve growth factor. Each monomer contains two unequal-sized domains, and
the active site is formed by the association of the two subunits. It is an example of a kinase that acts on an already-phosphorylated form,
creating a bisphosphorylated compound. Of all the enzymes in this
pathway it appears to be the one for which the least structural information
is available; note that the best structure determined to date was Phil
Evans's 2.4 Å structure from 1988, and there have not been many other
structures done. ADP acts as an allosteric activator on this enzyme as
well as being a product of the reaction.
It is an example of a kinase that acts on an already-phosphorylated form,
creating a bisphosphorylated compound. Of all the enzymes in this
pathway it appears to be the one for which the least structural information
is available; note that the best structure determined to date was Phil
Evans's 2.4 Å structure from 1988, and there have not been many other
structures done. ADP acts as an allosteric activator on this enzyme as
well as being a product of the reaction. This is a large and important enzyme, and structure determinations began
(unsuccessfully) more than 25 years ago. Some bacterial and yeast forms require
a divalent cation as a cofactor, but the eukaryotic aldolases do not. The
non-cationic forms proceed through an imine (Schiff-base) intermediate. The
enzyme is active on fructose 1-phosphate as well as its "standard" substrate,
fructose 1,6-bisphosphate; in this context it forms part of the catabolic
pathway by which fructose itself can be used as an energy and carbon source.
This is a large and important enzyme, and structure determinations began
(unsuccessfully) more than 25 years ago. Some bacterial and yeast forms require
a divalent cation as a cofactor, but the eukaryotic aldolases do not. The
non-cationic forms proceed through an imine (Schiff-base) intermediate. The
enzyme is active on fructose 1-phosphate as well as its "standard" substrate,
fructose 1,6-bisphosphate; in this context it forms part of the catabolic
pathway by which fructose itself can be used as an energy and carbon source. and for some reason abbreviated "TIM") is possibly the most efficient
enzyme known,
in terms of the rate acceleration afforded by the enzyme relative
to the uncatalyzed reaction. It is a tetrameric enzyme with a characteristic
structure in which alpha helical stretches alternate with beta strands such
that the beta strands curve around to form a barrel-like structure with
the helices outside. This structural motif appears in many other enzymes,
and has become known as a "TIM barrel."
and for some reason abbreviated "TIM") is possibly the most efficient
enzyme known,
in terms of the rate acceleration afforded by the enzyme relative
to the uncatalyzed reaction. It is a tetrameric enzyme with a characteristic
structure in which alpha helical stretches alternate with beta strands such
that the beta strands curve around to form a barrel-like structure with
the helices outside. This structural motif appears in many other enzymes,
and has become known as a "TIM barrel." responsible for the conversion of its substrate to
1,3-bisphosphoglycerate.
It resembles several other tetrameric NAD-dependent oxidoreductases, like
lactate dehydrogenase, alcohol dehydrogenase and malate dehydrogenase;
all have characteristic structures in the NAD-binding region known as
"Rossmann folds", after Michael Rossmann,
who first characterized this class of enzymes structurally.
The enzyme is somewhat allosteric.
responsible for the conversion of its substrate to
1,3-bisphosphoglycerate.
It resembles several other tetrameric NAD-dependent oxidoreductases, like
lactate dehydrogenase, alcohol dehydrogenase and malate dehydrogenase;
all have characteristic structures in the NAD-binding region known as
"Rossmann folds", after Michael Rossmann,
who first characterized this class of enzymes structurally.
The enzyme is somewhat allosteric. It is named for the reaction running in the opposite direction relative
the one shown in the chart and table above. In the direction shown in the
table it produces ATP rather than consuming it. This enzyme has been shown
to have a hinge motion about a point near the center of the molecule; the
open and closed forms of the enzyme involve movements as large as 17Å
in the residues farthest from the hinge point. This enzyme is primarily alpha-helical
in conformation.
It is named for the reaction running in the opposite direction relative
the one shown in the chart and table above. In the direction shown in the
table it produces ATP rather than consuming it. This enzyme has been shown
to have a hinge motion about a point near the center of the molecule; the
open and closed forms of the enzyme involve movements as large as 17Å
in the residues farthest from the hinge point. This enzyme is primarily alpha-helical
in conformation. According to Mathews's
textbook,
According to Mathews's
textbook,
 This reaction plays a role in gluconeogenesis as well as glycolysis.
Mg2+ ions are required for activity, at least in some forms of
the enzyme. Vertebrate genes code for two slightly different forms of the
monomer of enolase, alpha and beta. Most of the enolase in fetal tissue is
alpha-alpha; mature skeletal muscle contains beta-beta; some alpha-alpha remains
in smooth muscle tissue.
This reaction plays a role in gluconeogenesis as well as glycolysis.
Mg2+ ions are required for activity, at least in some forms of
the enzyme. Vertebrate genes code for two slightly different forms of the
monomer of enolase, alpha and beta. Most of the enolase in fetal tissue is
alpha-alpha; mature skeletal muscle contains beta-beta; some alpha-alpha remains
in smooth muscle tissue. It transfers a phosphate from phosphoenolpyruvate to ADP, producing pyruvate
and ATP. The reaction is essentially irreversible. Fructose 1,6-bisphosphate,
the substrate for the aldolase reaction, is an activator of this enzyme,
affording a level of control, known as "feed-forward activation,"
over glycolysis.
Four isozymes of pyruvate kinase are found in humans, derived from two genes.
The fetal (M1) isozyme is nonregulated;
the allosterically regulated forms predominant in adults.
It transfers a phosphate from phosphoenolpyruvate to ADP, producing pyruvate
and ATP. The reaction is essentially irreversible. Fructose 1,6-bisphosphate,
the substrate for the aldolase reaction, is an activator of this enzyme,
affording a level of control, known as "feed-forward activation,"
over glycolysis.
Four isozymes of pyruvate kinase are found in humans, derived from two genes.
The fetal (M1) isozyme is nonregulated;
the allosterically regulated forms predominant in adults.
 The enzyme that catalyzes this conversion, lactate dehydrogenase,
is a tetrameric, NAD-dependent enzyme with a molecular mass around
35kDA per subunit--that is,
it is distinctly similar to glyceraldehyde 3-phosphate dehydrogenase.
It catalyzes the reaction
The enzyme that catalyzes this conversion, lactate dehydrogenase,
is a tetrameric, NAD-dependent enzyme with a molecular mass around
35kDA per subunit--that is,
it is distinctly similar to glyceraldehyde 3-phosphate dehydrogenase.
It catalyzes the reaction